The Future of the Content Economy
beehiiv started with newsletters. Now, they’re reimagining the entire content economy.
On November 13, beehiiv’s biggest updates ever are dropping at the Winter Release Event.
For the people shaping the next generation of content, community, and media, this is an event you won’t want to miss.
Welcome to the 5 new subscribers who have joined Future Human since our last edition! Join 297 other leaders learning about the future of human health by subscribing here:
Hi friend,
Welcome back to Future Human! It seems I scheduled this recap incorrectly yesterday, so let’s try again today. I hope you like the name Vitals. With the format changes post-HLTH, we thought we should assign some fun names to differentiate the two Future Human products. The weekly headline recap newsletter will be Vitals and the now-monthly deep dives will be Workups. So this here is Vitals #2. Next month, we will be releasing the first deep dive since the renaming, but we will keep the number going as we have published >30 deep dives, so that will be Workup #32 (deep dive #32).
Thank you all for bearing with us as we make this the best product possible for all stakeholders and enthusiasts across health innovation.
With that said, let’s jump into the news.

Andrew’s Take
Pfizer may have read the post-COVID world totally wrong, but they will not miss out on obesity. As you may have gathered from your employer no longer pestering you about your lack of COVID booster, the CDC recently stopped universally recommending the vaccine to all. They now leave it up to patients and their doctors. This was not great for Pfizer as one could gather. Sales have dropped 25%, with Q3 revenue coming in just under $900M for the Comirnaty (their COVID-19 vaccine) compared to $1.16B last year. What they thought would be a permanent recurring source of revenue has begun to dry up with one CDC tweet.
This, however, would explain their aggressive leap into the world of GLP-1’s, where the new focus is on taking the weekly injectables we all know and “love” (Ozempic, Wegovy, Mounjaro, and Zepbound) and making them monthly. The leader in that race is not actually a big pharma incumbent, but rather a startup founded in 2022 as a joint venture between Population Health Partners and ARCH Venture Partners. Metsera boasts a lead asset (MET-097i) that is designed for only once-monthly injection. Metsera went public in January at a $2.7B valuation and has since ballooned to $7.5B.
Pfizer initially announced plans to acquire Metsera on September 22nd at a $4.9B valuation. That lasted all of a month before Metsera went back on the plan and announced another offer from Novo Nordisk (obesity drug giant and manufacturer of Ozempic and Wegovy) for ~$10B. Long story short, Pfizer sued and what ended up putting them back on top was two fold. First, they doubled their offer to $10B. Second, and arguably more important, the regulatory risk with the Novo Nordisk proved too high. You see, with Novo commanding 62% of the GLP-1 market in 2024 (42% in the U.S.), there was a high chance federal regulators would say the acquisition of Metsera would constitute monopolization and deny it. Given this risk, Metsera turned back to Pfizer who bumped up the offer by a casual 100%.
All in, if Pfizer innovates properly with their new asset, this could be a terrific example of competition working in the customer’s (patient’s) favor. Two giants fighting to create a better offering will hopefully do everyone some good across the obesity and cardiovascular spaces. Let’s wait and see.

Andrew’s Take
I love seeing a subject Future Human has gone deep on gain traction and make serious progress. Nervous system stimulation was the topic of both deep dive #11 (NeuroBionics) and #20 (Phantom Neuro). In both of those, within the Market section, we covered competitor Synchron, a leader in brain-computer interfaces (BCI). It seems they are still competing quite well.
As a refresher, their BCI allows paralyzed patients to control digital devices like other competing products do, but their implant can be placed on the motor cortex via the jugular vein, allowing for a minimally invasive, catheter-based process that avoids surgery. They raised $75 million last year from Bill Gates and Jeff Bezos and secured Breakthrough Device designation and Investigational Device Exemption in the years before that. Their device has been implanted in 10 patients and this summer they were the first to link to an iPad, Apple Vision Pro, and Amazon Alexa (think for turning on/off lights and changing music).
My read is that they are trying to power through trials by throwing infinite money at it. I don’t say that negatively, as in a capital-intensive sector such as medical devices (let alone brain implants) your cash runway needs to be robust and without potholes. They have the cash and will now use it to beef up their engineering and neuroscience teams in New York and San Diego to fill those potholes before takeoff. All aboard! Shoot, is that for boats?

Andrew’s Take
This Apple-Masimo debate has been going on for what feels like forever (sure enough it started in 2020). Well, it seems like it has gotten one step closer to concluding as a jury in California ruled last Friday that Apple must pay the medical device maker $634 million for patient infringement on the blood-oxygen monitoring technology they use in the Apple Watch. This is a core decision, but over the last five years it represents only one of 25 patents Masimo claims Apple infringed.
For the Apple Watch owners among us, this situation (especially the U.S. International Trade Commission’s first decision siding with Masimo in 2023) explains why your watches have not offered blood oxygen levels in many years. Unsurprisingly, Apple geniuses found a work around and announced in August the feature would be back as the readings now get measured on the paired iPhone instead of on the watch.
This amount of $634M is quite literally a water molecule, not even a drop, in the bucket of Apple’s revenue ($391B in 2024). That said, I do think it shows the little guy can hold their own in the U.S. legal system if they arrive with all their facts and have the endurance to fight for five years. It is key to mention that Masimo has not won all the suits before, so they are not necessarily the “right” side here but in this specific case they proved to be. I will be watching to see if they come after Apple again as the workarounds for blood oxygen go mainstream.
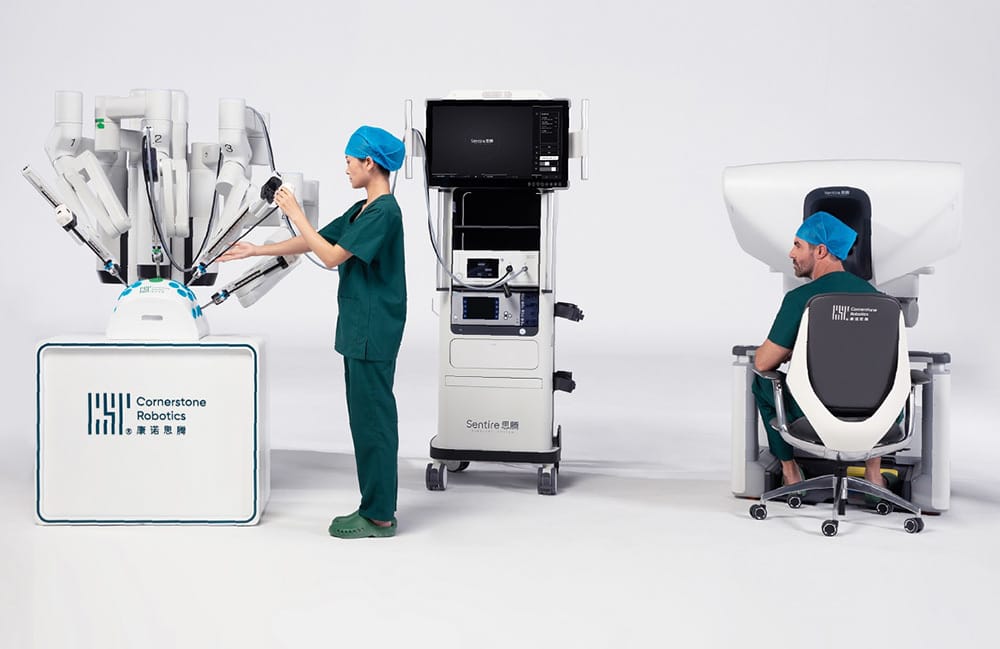
Andrew’s Take
Ahhhh, another Future Human topic of exploration. All the way back in March for deep dive #4, we wrote about CMR Surgical and its quest to take on Intuitive. As a reminder, Intuitive and its Da Vinci surgical robot command about 60% of the robotic surgery market. Since I wrote that newsletter, however, the competition has heated up and we have a new entrant out of Hong Kong. Cornerstone’s Sentire robot won approval for use in China in 2024 and is stepping up to enter the European market. Like we mentioned with Synchron above, the medical hardware timeline is long and expensive. This funding round will go toward accelerating their pace to market as investors are undoubtedly waiting for a return and hope this new injection speeds that up.
Although Cornerstone has not announced the price of their robot publicly, the commentary from surgeons who have used it makes it sound like it is the cheaper version of Da Vinci with almost all the same features. I gather they are taking the 90% the quality at 60% the price approach here. At >$2M, the Intuitive Da Vinci robot is not cheap, but it is gaining rapid traction across many areas of surgery previously hesitant to adopt the technique. Cornerstone will have an uphill battle, but with healthcare margins growing slimmer and slimmer I would not be surprised if hundreds of health systems jumped at the opportunity to purchase cheaper Da Vinci mimics that can run the same procedures.

Andrew’s Take
A slightly different angle here with less business news and more health research. A landmark study out of the University of Utah health system has begun to change our understanding of the neural basis of anxiety. It appears that key regulators not in neurons, but in the brain’s own immune cells, known as microglia, may control our anxiety. They found two types of microglia that seem to perform opposite functions: one being an accelerator to drive anxiety and the other being a brake. This naturally puts into question the decades long assumption that neural circuits regulate anxiety.
They found that non-Hoxb8 microglia alone caused mice to display classic signs of high anxiety, confirming their role as the 'gas pedal.' Conversely, Hoxb8 microglia acted as the 'brake pedal,' keeping anxiety levels normal. In a healthy brain, these two populations work together in opposition, with their balance setting the appropriate level of anxiety in response to the environment.
Though this is early stage and only in mouse models, I thought our Vitals newsletters could use at least one story that is merely a publication but still could change the future of medicine if validated longterm. Existing pharmaceutical interventions for anxiety disorders almost exclusively target neurons and their neurotransmitters. This work out of Utah will surely drive companies to now target brain immune cells with new therapeutics and see how anxiety levels respond. One in five Americans are impacted by anxiety, so to say this is a significant finding is an understatement of royal proportion.
We hope you enjoyed this edition of Vitals!
We always appreciate feedback, questions, and conversation, so feel free to reach out on LinkedIn or by replying to this email.
To more lives saved,
Andrew, Nicholas, and Isabelle



