Shoppers are adding to cart for the holidays
Over the next year, Roku predicts that 100% of the streaming audience will see ads. For growth marketers in 2026, CTV will remain an important “safe space” as AI creates widespread disruption in the search and social channels. Plus, easier access to self-serve CTV ad buying tools and targeting options will lead to a surge in locally-targeted streaming campaigns.
Read our guide to find out why growth marketers should make sure CTV is part of their 2026 media mix.
Welcome to the 4 new subscribers who have joined Future Human since our last edition! Join 321 other leaders learning about the future of human health by subscribing here:
Hi friend,
Welcome back to Future Human! I hope you are all enjoying the holiday season—and for those in the Northeast, the frigid temperatures! I’m starting my final week of preclinicals here in medical school, so I really have just one more week of traditional class left in my life. Insane—but I couldn’t be more excited to start clerkships.
It was nice to take a break from studying over the weekend to review the headlines and put this list together. We hope you enjoy!
To more lives saved,
Andrew, Nicholas, and Isabelle

Andrew’s Take
We have ourselves a rare medical device announcement saving both patient lives and the environment. Philips has for 7+ years now been innovating in the helium-free MRI space. In 2018 they removed helium in their 1.5T (Tesla) model. This week, they announced they figured it out in their stronger 3.0T edition. I can already anticipate two key questions:
What is the difference between 1.5T and 3.0T models?
Why does saving liquid helium matter?
Let’s get into it. The 3T MRI is a higher resolution option with faster scan times thanks to a stronger magnetic field (hence the 3.0 vs. 1.5T). It is therefore better for detailed imaging like neurological or vascular scans or for patients who have difficulty staying still. The 1.5T MRI is often sufficient for most patients and also offers a cheaper price tag and better suitability for patients with implants that may be affected by a stronger magnet.
As for helium, it exists underground with natural gas. The estimates of how much is on Earth vary wildly, but it is decidedly finite. At current rates of use, I saw estimates claiming we have a few decades left and others saying a bit more than a century. All are lower than we should be comfortable with given its critical use in medical equipment, rocket fuel pressurization, and breathing apparati. As for why it is used in MRIs, it has the lowest boiling point of any element (4 K), so it can stay much colder as a liquid to cool other objects (it has no freezing point at normal atmospheric pressure). In MRI machines, it keeps superconducting wires in the magnet cold enough to keep superconducting.
Philips came out with the BlueSeal technology to move away from this nonrenewable resource. Although the tech is properly underwraps, it seems helium-free systems use a sealed ecosystem where instead of a large helium bath, they contain a tiny amount of helium that never needs to be replaced. Seems “helium-free” refers to the fact that none is spent with each use. Other cooling strategies include copper-based heat exchangers. As a different approach, you do not need to cool as much if you do not get as hot, so Philips has also deployed artificial intelligence in this model to enhance image quality and accelerate scans without using the magnet as intensely read: less heat). Maybe soon they can get rid of the insanely loud clanking!

Andrew’s Take
Is anyone really satisfied with their insurance? You’re probably not and neither is your employer. Angle Health is scooping up boat loads of cash in order to fix that, and they are doing it with (shockingly) an AI-enabled insurance platform. Their system customizes plans for each employer based on real time data and trends. For small-to-medium-sized businesses, Angle will integrate medical and pharmacy data, consider demographic information of their workers, and track claims patterns to highlight risks among an employer's workforce before designing an intervention for them. It is living, breathing insurance.
They last raised in 2022, and have since 26x-ed their top line revenue (that was not a typo). They are available in 44 states and work with 3,000 employers. For these small and midsized employers, Angle makes the claim that healthcare benefit costs are outpacing inflation and with their AI-personalized plans, they can ensure money goes only where it needs to go to keep workers healthy. Few things have ever been able to really shift the insurance needle. AI may not be the silver bullet, but it appears Angle is at least deploying it responsibly and taking a promising approach to the monstrous industry. I am excited to follow this project and hope to see them save employers money while adequately caring for employees.

Andrew’s Take
Tech innovation is frequently painted as the wild west—absent of rules and filled with overzealous cowboys. While the second may be true, here in the U.S., the first is not always the case. The FDA and Whoop have been at it with regard to Whoop’s new blood pressure feature since May and it does not seem to be settling down. Normally, the FDA sends a warning letter as a courtesy, allowing the company to fix an issue noted by the federal agency. That company can also defend itself, but historically the FDA wins in court. The FDA can even escalate to the DOJ, gathering search warrants to seize the products of interest. While we are not at the second phase yet, Whoop does not appear to be backing down.
The “wellness’ category is ridiculously complex, filled with all types of health promises that often cannot be defended by evidence. A product used for general wellness must carry minimal safety risk and must not make any claims that mention a disease or condition. Interestingly, the 21st Century Cures Act that offers a lot of this framework does not provide real criteria for deciding whether something is wellness related or not. Therein lies one of the issues.
A second problem—other notable companies had to get full clearance just for blood pressure adjacent readings that are not as quantitative as Whoop. Apple, for example, which just this past September received clearance for hypertension notifications does not provide a pressure reading but rather a general warning that your values “may” be elevated. If that required a full clearance process, it seems sort of obvious that Whoop would have to as well. This case (Apple) is what most legal experts are saying will be the nail in the coffin for Whoop. They may soon need to seek clearance to offer the pressure values to users. Quite interesting legal precedents being set! Let us watch and see!

Andrew’s Take
Did not think I would be covering an Australian startup anytime soon, but (Crikey!) I’m excited. All G uses precision fermentation to create human and cow milk protein (lactoferrin), the same found in nature, in controlled tanks for all children who need the nutrition. They just announced a joint venture with French dairy expert Armor Proteines to commercialize the lactoferrin. The All G team is preparing for the launch of their first product, recombinant bovine lactoferrin, in the first quarter of 2026. Human edition will come later in the year. This round of funding comes before their Series B round as a means to support the joint venture, build distribution channels, and develop relationships with leaders in infant formula and adult nutrition/supplementation.
For those less well read on milk proteins, lactoferrin is an iron-binding anti-microbial protein that has been shown to improve immune function, iron homeostasis, GI health, and dermatological health. Its concentration in milk is low, so to traditionally isolate one kilogram demands thousands of liters of milk (therefore costly). All G believes that in their fermentation tanks they can use genetically prepared microbes to ferment lactoferrin. The challenges of achieving yields high enough for commercial use persist, but this funding will be a major boost to the efforts to overcome these barriers. As for their new partner, Armor Proteines is one of the world’s top producers of bovine lactoferrin, but even their supply cannot meet demand. This partnership should give them the technical assets to produce more and All G the best distribution and credibility to succeed. For the malnutritioned everywhere, I hope this fermentation technique takes off as soon as possible.
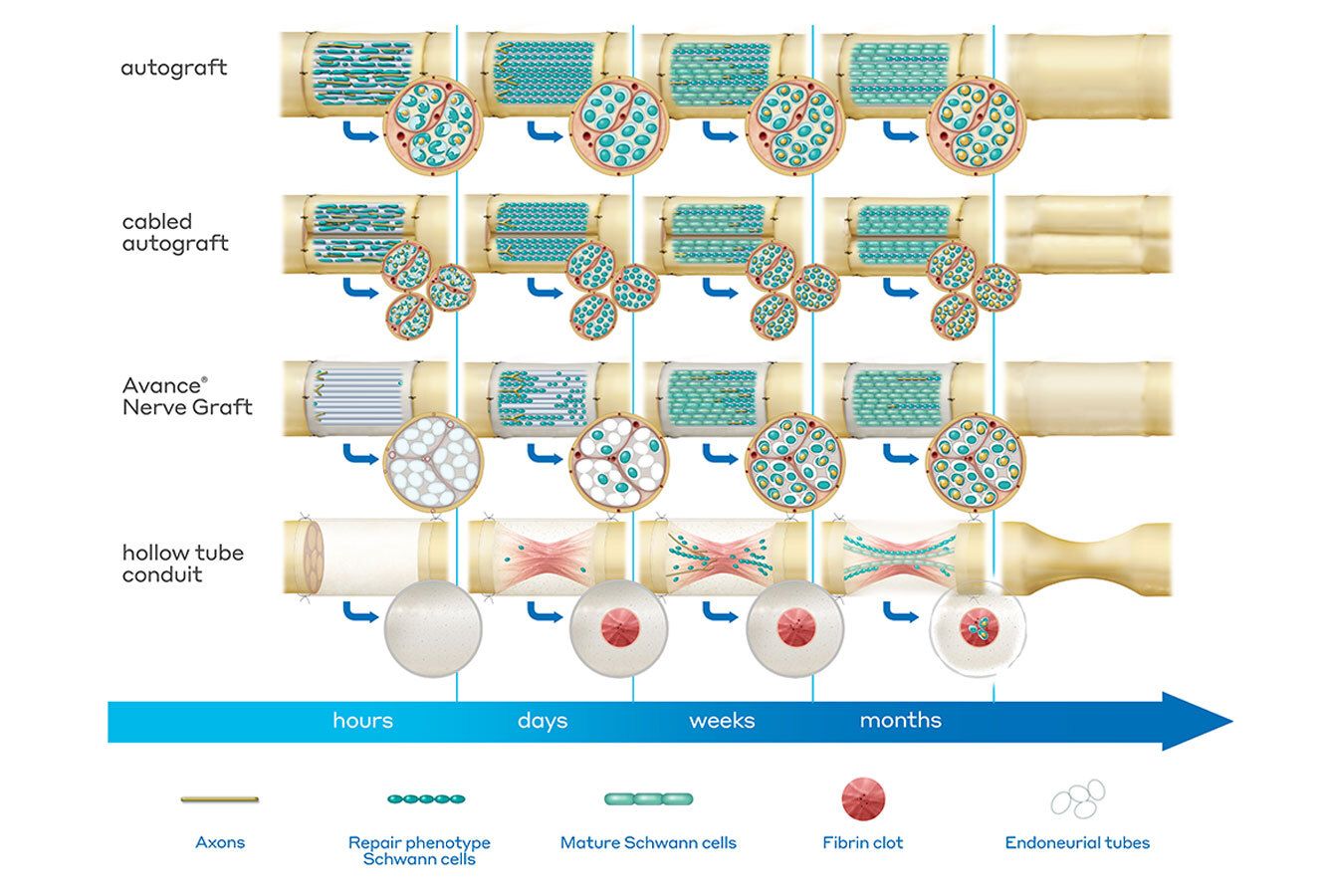
Andrew’s Take
Tissue engineering never fails to amaze me. Just the thought of building a scaffold that can be ‘installed’ to allow native cells to migrate and expand to new areas previously restricted is fascinating. Seems we have another big win in this space.
Axogen is expanding access for AVANCE® (acellular nerve allograft–arwx) — a product used to repair damaged peripheral nerves. The specific approval is for its “Biologics License Application (BLA)”. This means the FDA now treats AVANCE as a regulated biologic (rather than just donated tissue), confirming it meets standards for safety, quality, and effectiveness. As a result, AVANCE can now be more widely used — for adults and children (from 1 month old and up) — to fix sensory, motor, or mixed peripheral nerve injuries. The approval also expands the kinds of nerve injuries it can treat: even gaps larger than 25 mm or mixed/motor nerve damage are covered (some under an “Accelerated Approval” pathway, pending follow-up studies).
This is a major regulatory milestone. This kind of approval can accelerate adoption, increase insurance reimbursement likelihood, and ultimately make nerve repair a more standard-of-care option. Currently, end-to-end repairs where nerves are sutured together when cut and nerve grafts when the ends cannot meet without tension are standard of care.
We hope you enjoyed this edition of Vitals!
We always appreciate feedback, questions, and conversation, so feel free to reach out on LinkedIn or by replying to this email.
To more lives saved,
Andrew, Nicholas, and Isabelle



