Shoppers are adding to cart for the holidays
Over the next year, Roku predicts that 100% of the streaming audience will see ads. For growth marketers in 2026, CTV will remain an important “safe space” as AI creates widespread disruption in the search and social channels. Plus, easier access to self-serve CTV ad buying tools and targeting options will lead to a surge in locally-targeted streaming campaigns.
Read our guide to find out why growth marketers should make sure CTV is part of their 2026 media mix.
Welcome to the 10 new subscribers who have joined Future Human since our last edition! Join 331 other leaders learning about the future of human health by subscribing here:
Hi friend,
Welcome back to Future Human! I hope everyone is gearing up for an amazing holiday season wherever you are located. Here in NYC, the snow has been falling and it is looking to be a much colder Christmas than in previous years. I am actually heading out of the city shortly to enjoy my break from school with my family. I need to appreciate the time off while I can prior to clerkships.
I hope you all get some wonderful time with family this holiday season. Alright, let’s get to the headlines.
To more lives saved,
Andrew, Nicholas, and Isabelle
Andrew’s Take
We have all heard the warnings from our physicians or just generally AI-conscious citizens – be careful when turning to generative AI chatbots for medical advice and analysis. What ChatGPT, Gemini, or Copilot tell you is prone to error and even if accurate, can send you down rabbit holes that encourage over analysis and increase healthcare costs. If our physicians are thinking like this, so are the biggest names in AI and technology. Alphabet's Verily released its own AI-powered app in October that allows patients to connect their medical records and ask a chatbot their health-related questions. As we have written many times before, OpenAI has also made it clear with recent hires and product launches that they intend to dive into consumer health as well.
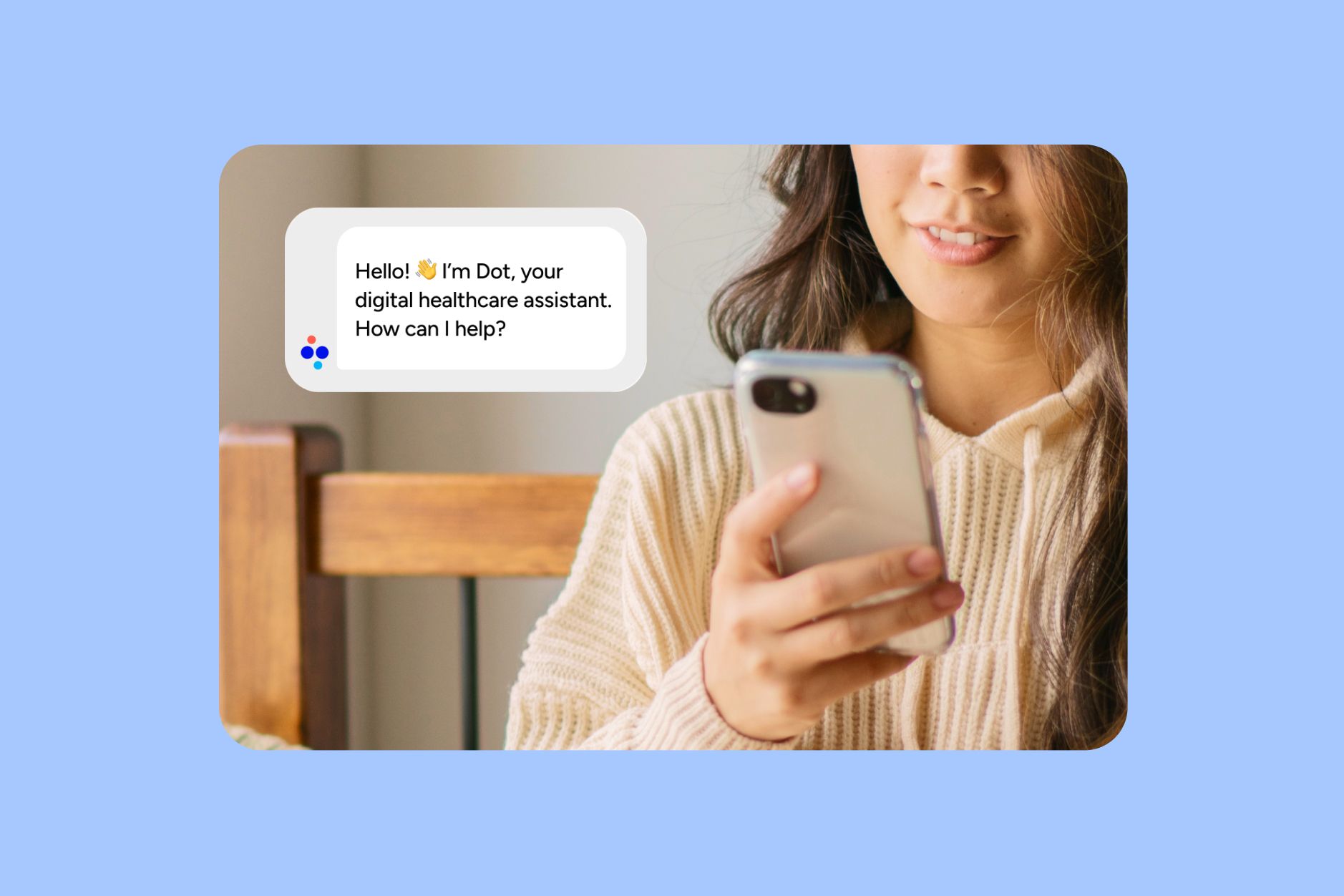
Included Health aims to take these behemoths on with their assistant, Dot, and move quicker thanks to their startup size. The team, which already sells products to personalize how patients interact with the medical system, has been testing Dot for 18 months to adjust before releasing to their entire base. It is one thing to help patients find key data points in the pages of records, but it is an entirely different thing to offer personalized medical advice. The regulatory maize expands drastically with just one move. Included’s C-suite makes it clear that their assistant is not meant to replace the clinician. They employ numerous clinicians and care advocates that members can speak with if they prefer. They maintain the position that the final team standing in this health assistant race will be the one employing physicians across all 50 states to deliver personalized care. This assistant is just meant to make the process of getting and understanding care easier. Let’s see what the regulators think.

Andrew’s Take
Neuralink just hired the former FDA official who most recently led approvals for neurological devices. If you’re looking for a “first hire” that matters, this is about as consequential as it gets.
David McMullen is heading to Neuralink, Elon Musk’s brain-computer-interface company looking to offer humans the ability to connect to computer-devices with just their thoughts. Most recently, McMullen was the director at the Food and Drug Administration’s Office of Neurological and Physical Medicine Devices, which handles review of BCI devices like Neuralink’s implant. In the BCI race, many teams are at or even ahead of Neuralink scientifically speaking, but none have as much capital. Musk’s group is coming off of a $650M Series E raised in June of this year, so no expense will be spared. They began human trials in 2024 after the FDA initially rejected their application in 2022. Since the trial started, 12 people worldwide with severe paralysis have gotten the implant and are using them to control digital tools with only thought. This rapid pace is not without its hiccups. Earlier this year, Neuralink came under fire from U.S. regulators for their animal testing program after employees said they were killing more animals than necessary. The rapid time pressure Musk put on them was allowing for critical mistakes and botched experiments.
Although some teams would look at this as an opportunity to step back and improve their process, Neuralink has instead decided to just bring on the guy in charge of overseeing them and decapitate the neurological regulatory board. This is also coming soon after the current administration has promised to end the revolving door of government regulators moving into private sectors to reveal secrets and help teams circumvent key rules and regulations.

Andrew’s Take
Imagine having to sell a software product to someone that will never interact with it in the ideal setting. That is what Valerie Health CEO Peter Shalek and co-founder Nitin Joshi are tasked with everyday when facing physicians. Valerie aims to fully automate the front-office tasks in healthcare (think referrals and scheduling). Somehow, they are handling the challenge quite well as they are expanding rapidly and just raised $30M to keep it going.
Valerie Health seems to specifically be targeting the country’s largest independent provider groups. In their eyes, juggling patient intakes creates backlogs of referrals and the associated administrative/financial burdens lead to provider groups being acquired by hospitals or private equity firms. This move tends to consolidate care, drive up costs, and lower satisfaction amongst patients. What if the administrative burden could be wiped out with one SaaS purchase?
The space is not without its competition. Andreessen Horowitz-backed Tennr recently raised $101M in a Series C to automate referrals. To keep up, Valerie intends to deploy the capital in order to snag as many customers as possible next year. As Epic has shown, once a healthcare organization integrates a new software platform, the relationship becomes extremely sticky — displacing the incumbent is often close to impossible. Valerie has seen revenue triple last quarter from the previous quarter (not even the last year), which is quite impressive. They are predicting revenue to grow 6x next year. Let us see how they continue to compete in this healthcare admin AI battleground.

Andrew’s Take
The FDA has taken a big leap and backed Flow Neuroscience’s FL-100 for at-home brain stimulation to treat major depressive disorder (MDD) in adults. This is the first device of its kind approved fro at-home use. The phase 2 trial, called the Empower study, was a multi-center, double-blind, randomized 20-week trial including 174 subjects. Patients had a diagnosis of unipolar major depressive disorder according to the DSM-5, had a Hamilton Depression Rating Score (HDRS) of 16 or higher, and were allowed antidepressant or psychotherapy regimens as long as they were stable for 6 weeks prior to study enrollment. The results showed 58% of patients achieved remission of their depression after 10 weeks. Among global users, Flow Neuroscience has shared that 77% report improvement in symptoms within 3 weeks. Although this is a major approval, the product has already been used by 55,000+ patients across Europe and Hong Kong.
Future Human has written a couple deep dives about brain stimulation devices looking to improve all sorts of disease states from depression to epilepsy. I am amazed we have not covered Flow yet. Their device is worn as a headset and delivers transcranial direct current stimulation to the dorsolateral prefrontal cortex, a brain region associated with mood. Typical treatment is a 12-week course, with 5 sessions per week for the first 3 weeks, tapering to 2 to 3 sessions weekly for the last 9 weeks. Individual treatment sessions with the brain-stimulation device are 30 minutes. In the U.S., they plan to launch the device in the 2nd quarter of 2026, available as a prescription-only treatment. The US retail price is estimated to be between $500 and $800, with insurance coverage details being announced in early 2026. I believe the at-home approval is arguably the most significant for this company’s success. I have no doubt access and uptake will skyrocket when patients with MDD learn they do not need to go into a physician’s office to receive their regular treatment. Putting control back in the hands of the patients is arguably most important in the mental health arena. I am thrilled to see the FDA supporting that.

Andrew’s Take
South Korean neurodegenerative disease biotech ADEL, Inc. has inked a major global licensing agreement with French pharmaceutical giant Sanofi valued at up to $1.04 billion. Under the deal, Sanofi gains exclusive worldwide rights to develop and commercialize ADEL-Y01, a novel antibody therapy targeting a specific pathological form of the tau protein implicated in Alzheimer’s disease. ADEL will receive an $80 million upfront payment, with additional milestone payments and tiered royalties tied to clinical and commercial achievements.
The Alzheimer’s space is a brutal one to say the least. Few in the biotech/pharma space can forget Aduhelm (aducanumab), Biogen’s controversial Alzheimer’s drug that failed commercially and was ultimately discontinued after major market pushback. Whenever I see another pharma behemoth leap into the space, I am excited for the patients but hesitant on the science. ADEL-Y01 is designed to selectively bind acetylated tau at lysine-280 (acK280), a modified form of tau that aggregates and contributes to neural toxicity, while sparing normal tau function — a strategy that may offer a differentiated mechanism compared with broader tau-targeted therapies currently in development. The asset is already being evaluated in a Phase 1 first-in-human trial in the United States under an FDA-cleared Investigational New Drug (IND) application.
For ADEL, the partnership provides developmental and commercial muscle to scale ADEL-Y01 globally. For Sanofi, this deal lines up with its broader strategic push in neurology and Alzheimer’s pipelines. I hope this pair is the one to break the curse and deliver a meaningful product to patients worldwide.
We hope you enjoyed this edition of Vitals!
We always appreciate feedback, questions, and conversation, so feel free to reach out on LinkedIn or by replying to this email.
To more lives saved,
Andrew, Nicholas, and Isabelle



