Easy setup, easy money
Making money from your content shouldn’t be complicated. With Google AdSense, it isn’t.
Automatic ad placement and optimization ensure the highest-paying, most relevant ads appear on your site. And it literally takes just seconds to set up.
That’s why WikiHow, the world’s most popular how-to site, keeps it simple with Google AdSense: “All you do is drop a little code on your website and Google AdSense immediately starts working.”
The TL;DR? You focus on creating. Google AdSense handles the rest.
Start earning the easy way with AdSense.
Welcome to the 2 new subscribers who have joined Future Human since our last edition! Join 333 other leaders learning about the future of human health by subscribing here:
Hi friend,
Welcome back to Future Human! I am writing to you all from New Jersey in the midst of a gorgeous snowstorm. It is absolutely dumping outside and it is great to watch while putting these stories and takeaways together. Although I can’t get much skiing in this year with school/rotations, I hope all of you east coast skiers get out there a bunch this winter because it is looking to be a wonderful season.
On a separate note, we have some insane healthtech headlines right before Christmas. It seems the FDA was in a holiday rush to deliver yeses and noes before many sit down and turn off their laptops and phones. A couple were in the cardiac space, so I can’t wait to tell you all about them. Alright, let’s get into it.
To more lives saved,
Andrew, Nicholas, and Isabelle

Andrew’s Take
It appears the Danes have bested the Americans in the race to weight loss convenience. Ever since the Ozempic/Wegovy/Mounjaro (read: GLP-1) craze began, the conversation has never failed to mention the ultimate goal of one day offering an oral option of this miracle weight loss drug. When I say miracle, it is not fully in jest, as few other drugs have racked up such an extensive indication profile in such a short period of time. We are talking: type 2 diabetes, chronic weight management/obesity, cardiovascular risk reduction, prediabetes, metabolic syndrome, polycystic ovary syndrome (PCOS), nonalcoholic fatty liver disease (NAFLD / NASH), and potentially heart failure with preserved ejection fraction and obstructive sleep apnea. The data keeps showing these molecules to be truly multifaceted assets. Now, Wegovy just took a leap ahead.
Novo Nordisk, which if you didn't know was formed in 1989 after the merger of rival Danish insulin companies Novo Terapeutisk Laboratorium and Nordisk Insulin Laboratorium, just announced they have achieved the first FDA approval for an oral GLP-1. Data from their pivotal OASIS 4 trial showed the Wegovy pill (semaglutide 25 mg once daily) led to a mean weight loss of 16.6% in 307 adults with obesity or overweight with one or more comorbidities. They now plan to launch the product in the US in early January 2026. Why the rush? Well Eli Lilly has all the same ideas with their oral GLP-1 drug (orforglipron) showing promising results from the ATTAIN-MAINTAIN trial earlier this year and now awaiting FDA review (submitted earlier this month). Who secures physician and patient loyalty first will have a massive leg up.
I have so many questions and am excited to watch what happens next. Will the FDA rush to review Eli Lilly’s product sooner? What happens to telehealth companies who built massive businesses on compounded (injectable) alternatives of the GLP-1 products in shortage? Let’s wait and see.

Andrew’s Take
Let’s continue with the FDA holiday presents. The notoriously bureaucratic federal agency has delivered a resounding ‘yes’ three days earlier than planned for Cytokinetics’ hypertrophic cardiomyopathy (HCM) treatment, Myqorzo (aficamten). For context, HCM is a genetic condition where the heart muscle — most often the wall between the chambers — becomes abnormally thick and stiff. This thickening can make it difficult for the heart to fill with or pump out enough blood, occasionally leading to serious complications like heart failure or dangerous heart rhythm.
As someone who is interested in cardiology, cardiothoracic surgery, and health innovation, this was the coolest headline I have come across all month. Cytokinetics was founded in 1997 and achieved their first FDA approval this week. Now, that is not to say they haven't done much in nearly 30 years. They are entering a market already occupied by a drug they helped create — Bristol Myers Squibb’s Camzyos. A 2012 partnership between Cytokinetics and MyoKardia led to the development of Camzyos. In 2020, BMS bought out MyoKardia for $13.1 billion and secured approval for Camzyos in 20222.
Myqorzo is a cardiac myosin inhibitor like Camzyos. It binds to the protein myosin and blocks the interactions which cause HCM, reducing cardiac contractions and obstruction in the left ventricle outflow tract (LVOT). Current HCM treatments revolve around symptom management, like beta-blockers and calcium channel blockers to slow the heart rate and reduce the force of contraction. Besides Camzyos, treatment at the source comes in the form of surgery (septal myectomy) where a cardiac surgeon removes a small piece of the thickened heart muscle (the septum) that is blocking the blood flow out of the heart.
Myqorzo is riding high against Camzyos though with a safer drug profile requiring no drug-drug interaction monitoring or complicated dosing. Will that push them ahead immediately?
Andrew’s Take
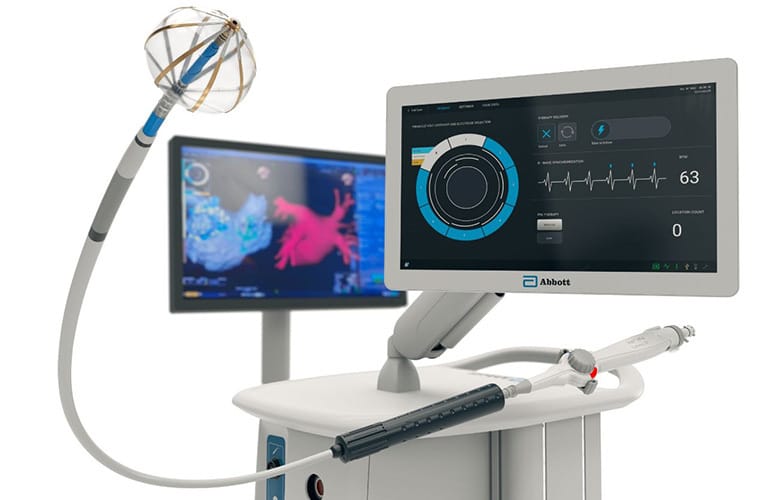
A team in my lab is working on advancing this exact technology, so this was a great headline to read. Abbott has been allowed to throw its hat in the atrial fibrillation (AFib) race. AFib is the world’s most common arrhythmia, causing the heart's upper chambers to quiver chaotically instead of beating effectively, causing an irregular and often rapid heartbeat that can increase the risk of blood clots and stroke. We understand well where this electrical malformation originates, and have for a long time been able to go in and burn or freeze the originating tissue to reset the electrical pacing. That, however, is not selective for the tissue of interest and can burn or freeze parts we did not aim to hit, leading to nerve palsies or fistulas. Enter Pulsed Field Ablation (PFA). PFA uses targeted electrical pulses for electroporation of heart cells, sparing other tissues because of different vulnerability thresholds.
Abbott is entering the PFA market with their Volt system, now approved by the FDA. They will soon be competing directly with Medtronic, Boston Scientific, and Johnson & Johnson MedTech in the US market (they received European CE mark approval earlier this year).
Volt lets physicians map, pace, and ablate, all within the same catheter (other devices require 2+ pieces). Abbot claims the single catheter approach improves workflow and adds flexibility. They matched a balloon-in-basket catheter with their EnSite X EP heart mapping system. The Ensite X helps physicians visualize and position tools in the heart. The simplified process also means patients can now avoid general anesthesia, being able to select conscious sedation if they prefer with the shorter procedure and faster recovery. Incredible step in the right direction.
This approval also came ahead of expectations (projected first half 2026). What has gotten into the FDA?

Andrew’s Take
Okay, I will stop lauding the FDA after this story. Sorry, I am just impressed with their end of year rush (maybe they just procrastinated that hard?).
Intuitive is not letting competitors get ahead in the single port arena. Their da Vinci SP (single port) system just got the okay to handle inguinal hernia repairs, gallbladder removals, and appendectomy procedures. The single port, as you can probably deduce, enables surgeons to perform procedures through a single incision or natural orifice, offering simplicity and precision. Despite the single port, surgeons can still control three multi-jointed instruments and an articulating 3DHD endoscope through the entry point. The single port advantage is not just fewer scars and improved patient recovery. The system allows for access to narrower and deeper spaces within the body to handle more complex procedures.
The SP system is still relatively new. Of the more than 1,790 systems Intuitive placed last year, about 95 were single port. More than 290 da Vinci SPs are installed in hospitals globally at the end of 2024, compared with more than 9,890 da Vinci multiport systems. New but all the more exciting.
This is not the first win for the SP this year. In May, Intuitive picked up clearance for transanal local excision/resection in colorectal procedures. They will need to keep racking these up as competition is advancing. Medtronic got the first FDA clearance for their Hugo robot earlier this year.
This approval is definitely a win, but I still can’t wrap my head around the valuation. I’ve probably said this before, but Intuitive’s multiple has honestly surprised me for years now. I get it—they dominate the market, maybe even to a monopolistic level—but it feels like investors are completely shrugging off the competition that’s clearly coming. A 76x P/E for a medtech company is wild when the sector typically trades closer to 30–40x. That’s a lot of optimism baked in. What do I know though?

Andrew’s Take
Okay, last one before we head off to Christmas! In the medtech world, I love when large incumbents make genuinely bold moves. Medtronic’s decision to spin out its diabetes business is one of those moments. MiniMed is filing for an IPO.
California-based MiniMed offers everything diabetes from insulin delivery devices to glucose monitors. It was founded in 1983 by Alfred Mann and bought by Medtronic in a nearly $3.3 billion deal in 2001. Medtronic is now spinning off MiniMed, partly because it's been a struggling, lower-margin area with quality/regulatory challenges. Think recalls and FDA warnings that dragged on Medtronic's overall performance. That said, its not all negative. The separation aims to unlock focused growth, allowing MiniMed independence for tailored investment and Medtronic to streamline its core. MiniMed has also recently shown double-digit growth, so perhaps the move frees both entities for better focus.
I am still trying to learn about the logic behind a spinoff, but it seems when a company separates a core division, it often signals that the unit hasn’t been performing the way leadership or public markets want. The diabetes business has certainly faced margin pressure, operational complexity, and increasing competition. Spinning it off simplifies Medtronic’s story (something Johnson & Johnson has been doing recently with its announcement to sell the orthopedic business). Medtronic will now refocus on higher-margin, more predictable segments. For MiniMed, this is also an admission that the unit may have struggled to compete effectively while buried inside a massive conglomerate. Independence might help, but it also exposes the business fully to public scrutiny and execution risk. An IPO doesn’t erase regulatory challenges, cost structure issues, or years of competitive catch-up. So while the spin-off creates opportunity, it also reads like Medtronic acknowledging that diabetes no longer fits cleanly into its long-term growth narrative. We shall watch closely.
We hope you enjoyed this edition of Vitals!
We always appreciate feedback, questions, and conversation, so feel free to reach out on LinkedIn or by replying to this email.
Merry Christmas,
Andrew, Nicholas, and Isabelle